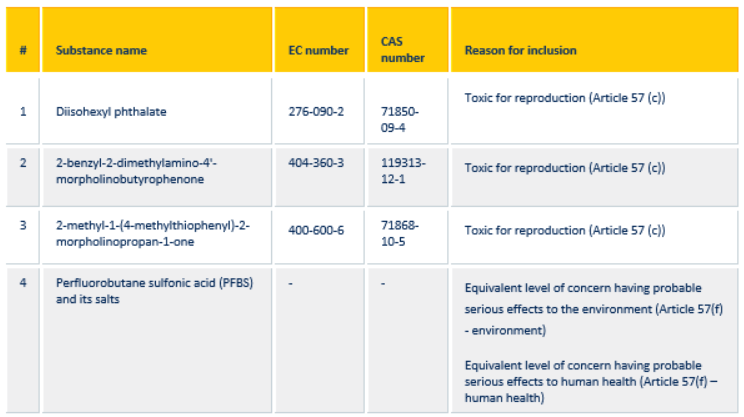
| # |
Substance name |
Intrinsic property(ies) referred to in Article 57 |
Latest application date |
Sunset date |
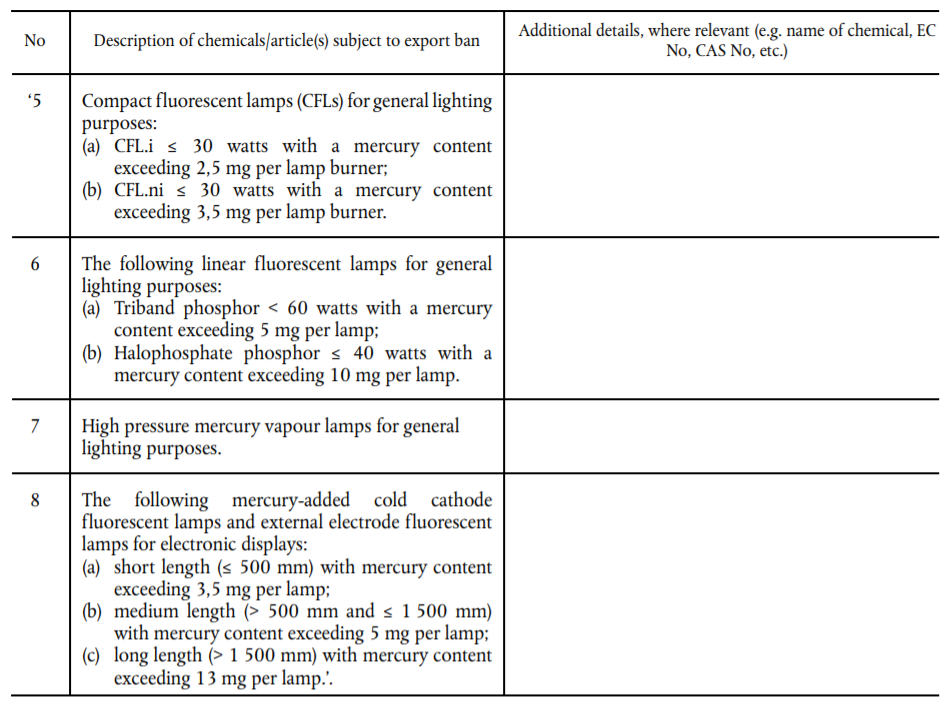
| 1 |
5-tert-butyl-2,4,6-trinitro-m-xylene (Musk xylene)
EC No: 201-329-4
CAS No: 81-15-2 |
vPvB |
21/02/2013 |
21/08/2014 |
| 2 |
4,4’- Diaminodiphenylmethane (MDA)
EC No: 202-974-4
CAS No: 101-77-9 |
Carcinogenic |
21/02/2013 |
21/08/2014 |
| 3 |
Hexabromocyclododecane (HBCDD)
and all major diastereoisomers identified |
PBT |
21/02/2014 |
21/08/2015 |
| 4 |
Bis(2-ethylhexyl) phthalate (DEHP)
EC No: 204-211-0
CAS No: 117-81-7 |
Toxic for reproduction |
21/08/2013 |
21/02/2015 |
| 5 |
Benzyl butyl phthalate (BBP)
EC No: 201-622-7
CAS No: 85-68-7 |
Toxic for reproduction |
21/08/2013 |
21/02/2015 |
| 6 |
Dibutyl phthalate (DBP)
EC No: 201-557-4
CAS No: 84-74-2 |
Toxic for reproduction |
21/08/2013 |
21/02/2015 |
| 7 |
Diisobutyl phthalate (DIBP)
EC No: 201-553-2
CAS No: 84-69-5 |
Toxic for reproduction |
21/08/2013 |
21/02/2015 |
| 8 |
Diarsenic trioxide
EC No: 215-481-4
CAS No: 1327-53-3 |
Carcinogenic |
21/11/2013 |
21/05/2015 |
| 9 |
Diarsenic pentaoxide
EC No: 215-116-9
CAS No: 1303-28-2 |
Carcinogenic |
21/11/2013 |
21/05/2015 |
| 10 |
Lead chromate
EC No: 231-846-0
CAS No: 7758-97-6 |
Carcinogenic
Toxic for reproduction |
21/11/2013 |
21/05/2015 |
| 11 |
Lead sulfochromate yellow
EC No: 215-693-7
CAS No: 1344-37-2 |
Carcinogenic
Toxic for reproduction |
21/11/2013 |
21/05/2015 |
| 12 |
Lead chromate molybdate sulfate red
EC No: 235-759-9
CAS No: 12656-85-8 |
Carcinogenic
Toxic for reproduction |
21/11/2013 |
21/05/2015 |
| 13 |
Tris(2-chloroethyl) phosphate
EC No: 204-118-5
CAS No: 115-96-8 |
Toxic for reproduction |
21/02/2014 |
21/08/2015 |
| 14 |
2,4-dinitrotoluene (2,4-DNT)
EC No: 204-450-0
CAS No: 121-14-2 |
Carcinogenic |
21/02/2014 |
21/08/2015 |
| 15 |
Trichloroethylene
EC No: 201-167-4
CAS No: 79-01-6 |
Carcinogenic |
21/10/2014 |
21/04/2016 |
| 16 |
Chromium trioxide
EC No: 215-607-8
CAS No: 1333-82-0 |
Carcinogenic
Mutagenic |
21/03/2016 |
21/09/2017 |
| 17 |
Acids generated from chromium trioxide and their oligomers |
Carcinogenic |
21/03/2016 |
21/09/2017 |
| 18 |
Sodium dichromate
EC No: 234-190-3
CAS No: 10588-01-9, 7789-12-0 |
Carcinogenic
Mutagenic
Toxic for reproduction |
21/03/2016 |
21/09/2017 |
| 19 |
Potassium dichromate
EC No: 231-906-6
CAS No: 7778-50-9 |
Carcinogenic
Mutagenic
Toxic for reproduction |
21/03/2016 |
21/09/2017 |
| 20 |
Ammonium dichromate
EC No: 232-143-1
CAS No: 7789-09-5 |
Carcinogenic
Mutagenic
Toxic for reproduction |
21/03/2016 |
21/09/2017 |
| 21 |
Potassium chromate
EC No: 232-140-5
CAS No: 7789-00-6 |
Carcinogenic
Mutagenic |
21/03/2016 |
21/09/2017 |
| 22 |
Sodium chromate
EC No: 231-889-5
CAS No: 7775-11-3 |
Carcinogenic
Mutagenic
Toxic for reproduction |
21/03/2016 |
21/09/2017 |
| 23 |
Formaldehyde, oligomeric reaction products with aniline
EC No: 500-036-1
CAS No: 25214-70-4 |
Carcinogenic |
22/02/2016 |
22/08/2017 |
| 24 |
Arsenic acid
EC No: 231-901-9
CAS No: 7778-39-4 |
Carcinogenic |
22/02/2016 |
22/08/2017 |
| 25 |
Bis(2-methoxyethyl) ether
EC No: 203-924-4
CAS No: 111-96-6 |
Toxic for reproduction |
22/02/2016 |
22/08/2017 |
| 26 |
1,2-dichloroethane (EDC)
EC No: 203-458-1
CAS No: 107-06-2 |
Carcinogenic |
22/05/2016 |
22/11/2017 |
| 27 |
2,2′-dichloro-4,4′-methylenedianiline (MOCA)
EC No: 202-918-9
CAS No: 101-14-4 |
Carcinogenic |
22/05/2016 |
22/11/2017 |
| 28 |
Dichromium tris(chromate)
EC No: 246-356-2
CAS No: 24613-89-6 |
Carcinogenic |
22/07/2017 |
22/01/2019 |
| 29 |
Strontium chromate
EC No: 232-142-6
CAS No: 7789-06-2 |
Carcinogenic |
22/07/2017 |
22/01/2019 |
| 30 |
Potassium hydroxyoctaoxodizincatedichromate
EC No: 234-329-8
CAS No: 11103-86-9 |
Carcinogenic |
22/07/2017 |
22/01/2019 |
| 31 |
Pentazinc chromate octahydroxide
EC No: 256-418-0
CAS No: 49663-84-5 |
Carcinogenic |
22/07/2017 |
22/01/2019 |
| 32 |
1-bromopropane (n-propyl bromide)
EC No: 203-445-0
CAS No: 106-94-5 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 33 |
Diisopentyl phthalate
EC No: 210-088-4
CAS No: 605-50-5 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 34 |
1,2-Benzenedicarboxylic acid, di-C6-8-branched alkyl esters, C7-rich
EC No: 276-158-1
CAS No: 71888-89-6 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 35 |
1,2-Benzenedicarboxylic acid, di-C7-11-branched and linear alkyl esters
EC No: 271-084-6
CAS No: 68515-42-4 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 36 |
1,2-Benzenedicarboxylic acid, dipentyl ester, branched and linear
EC No: 284-032-2
CAS No: 84777-06-0 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 37 |
Bis(2-methoxyethyl) phthalate
EC No: 204-212-6
CAS No: 117-82-8 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 38 |
Dipentyl phthalate
EC No: 205-017-9
CAS No: 131-18-0 |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 39 |
n-pentyl-isopentylphthalate
EC No: 933-378-9
CAS No: – |
Toxic for reproduction |
04/01/2019 |
04/07/2020 |
| 40 |
Anthracene oil
A complex combination of polycyclic aromatic hydrocarbons obtained from coal tar having an approximate distillation range of 300°C to 400°C (572°F to 752°F). Composed primarily of phenanthrene, anthracene and carbazole.
EC No: 292-602-7
CAS No: 90640-80-5 |
Carcinogenic
PBT
vPvB |
04/04/2019 |
04/10/2020 |
| 41 |
Pitch, coal tar, high-temp.
The residue from the distillation of high temperature coal tar. A black solid with an approximate softening point from 30°C to 180°C (86°F to 356°F). Composed primarily of a complex mixture of three or more membered condensed ring aromatic hydrocarbons.
EC No: 266-028-2
CAS No: 65996-93-2 |
Carcinogenic
PBT
vPvB |
04/04/2019 |
04/10/2020 |
| 42 |
4-(1,1,3,3-tetramethylbutyl)phenol, ethoxylated
covering well-defined substances and UVCB substances, polymers and homologues |
Endocrine disrupting properties |
04/07/2019 |
04/01/2021 |
| 43 |
4-Nonylphenol, branched and linear, ethoxylated
substances with a linear and/or branched alkyl chain with a carbon number of 9 covalently bound in position 4 to phenol, ethoxylated covering UVCB- and well-defined substances, polymers and homologues, which include any of the individual isomers and/or combinations thereof |
Endocrine disrupting properties |
04/07/2019 |
04/01/2021 |
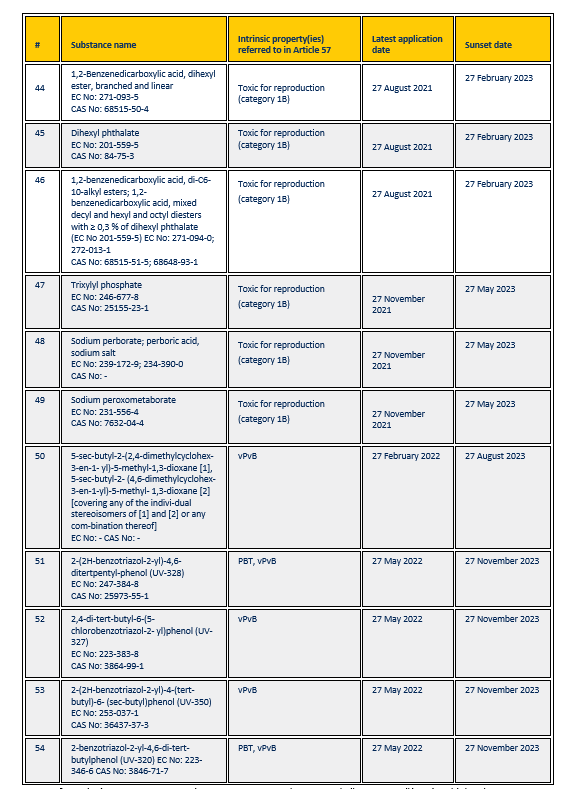
| 44 |
1,2-Benzenedicarboxylic acid, dihexyl ester, branched and linear
EC No: 271-093-5
CAS No: 68515-50-4 |
Toxic for reproduction (category 1B) |
27 August 2021 |
27 February 2023 |
| 45 |
Dihexyl phthalate
EC No: 201-559-5
CAS No: 84-75-3 |
Toxic for reproduction (category 1B) |
27 August 2021 |
27 February 2023 |
| 46 |
1,2-benzenedicarboxylic acid, di-C6-10-alkyl esters; 1,2-benzenedicarboxylic acid, mixed decyl and hexyl and octyl diesters with ≥ 0,3 % of dihexyl phthalate
(EC No 201-559-5) EC No: 271-094-0; 272-013-1
CAS No: 68515-51-5; 68648-93-1 |
Toxic for reproduction (category 1B) |
27 August 2021 |
27 February 2023 |
| 47 |
Trixylyl phosphate
EC No: 246-677-8
CAS No: 25155-23-1 |
Toxic for reproduction (category 1B) |
27 November 2021 |
27 May 2023 |
| 48 |
Sodium perborate; perboric acid, sodium salt
EC No: 239-172-9; 234-390-0
CAS No: – |
Toxic for reproduction (category 1B) |
27 November 2021 |
27 May 2023 |
| 49 |
Sodium peroxometaborate
EC No: 231-556-4
CAS No: 7632-04-4 |
Toxic for reproduction (category 1B) |
27 November 2021 |
27 May 2023 |
| 50 |
5-sec-butyl-2-(2,4-dimethylcyclohex-3-en-1- yl)-5-methyl-1,3-dioxane [1], 5-sec-butyl-2- (4,6-dimethylcyclohex-3-en-1-yl)-5-methyl- 1,3-dioxane [2] [covering any of the individual stereoisomers of [1] and [2] or any combination thereof]
EC No: – CAS No: – |
vPvB |
27 February 2022 |
27 August 2023 |
| 51 |
2-(2H-benzotriazol-2-yl)-4,6-ditertpentylphenol (UV-328)
EC No: 247-384-8
CAS No: 25973-55-1 |
PBT, vPvB |
27 May 2022 |
27 November 2023 |
| 52 |
2,4-di-tert-butyl-6-(5-chlorobenzotriazol-2- yl)phenol (UV-327)
EC No: 223-383-8
CAS No: 3864-99-1 |
vPvB |
27 May 2022 |
27 November 2023 |
| 53 |
2-(2H-benzotriazol-2-yl)-4-(tert-butyl)-6- (sec-butyl)phenol (UV-350) EC No: 253-037-1
CAS No: 36437-37-3 |
vPvB |
27 May 2022 |
27 November 2023 |
| 54 |
2-benzotriazol-2-yl-4,6-di-tert-butylphenol (UV-320) EC No: 223-346-6 CAS No: 3846-71-7 |
PBT, vPvB |
27 May 2022 |
27 November 2023 |


![]()